Vector transmission model¶
The vector model can be run in one of two modes: as a cohort model or as an individual mosquito model (see Malaria model overview for more information). The vector model inherits the same human-infection model structure from the generic simulation type: uninfected, latent incubation, infectious, multiple immune variables, and super-infection. However, the transmission of infections is not between individual humans, but rather via the human-to-vector and vector-to-human pathways. The model is a closed-loop feeding cycle where a successful vector (mosquito) feeds (both indoors and outdoors), mates, then produce eggs which become larva and then adult mosquitoes.
For example, vector blood-feeding branches into various probabilities that are calculated once per time step. These calculations are based on species parameters and aggregated vector interventions (see feeding decision tree).
Vector transmission can be thought of in terms of vector ecology, and in the EMOD framework is comprised of several components: 1. Vector tracking and the mosquito life-cycle, 2. mosquito behavior, which involves feeding (see Modeling feeding cycle outcomes), and 3. transmission of malaria-causing Plasmodium parasites between humans and mosquitoes (see Transmission between humans and vectors). Each of these components is described in more detail below.
For a complete list of configuration parameters that are used in the malaria model, see the Configuration parameters.
Vector tracking and the mosquito life-cycle¶
Populations are tracked as cohorts throughout the full mosquito life-cycle:
From eggs to larvae with a temperature-dependent development period preceding emergence
A brief immature phase including sugar-feeding and mating
Repeating multi-day gonotrophic cycles during which mosquitoes may be exposed to infection by gametocytes
A temperature-dependent latency for sporogony
Life-cycle progression is modulated through a set of required species-specific parameters.
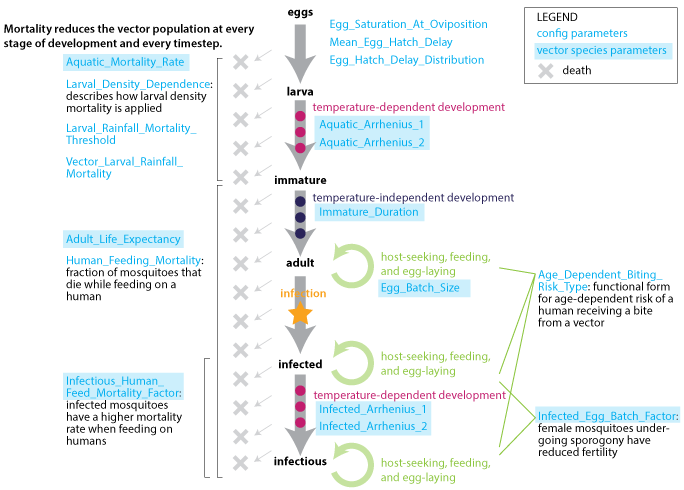
Vector life cycle¶
Modeling feeding cycle outcomes¶
Adult vectors enter a cycle of host-seeking, feeding, and egg-laying that continues until death. Successful vector blood-feeding branches into various probabilities that are calculated once per time step. These calculations are based on species parameters (for example, An. arabiensis has a higher propensity to feed outdoors or on livestock) and aggregated vector interventions.
Various feeding cycle outcomes are calculated from branching trees of conditional probabilities, and individual interventions modulate the probability of choosing between branches. Feeding cycle outcomes include:
Death before, during, or after feeding
Host unavailable
Successful human feed
The allocation of mosquitoes to feeding-cycle outcomes is based on end-state probabilities that have been aggregated over the individual humans in the simulation.
Multiple simultaneous interventions can target various branches in the vector feeding tree. For example, when both indoor residual spraying (IRS) and insecticide-treated nets (ITN) are applied against indoor host-seeking mosquitoes, IRS can discourage mosquitoes from entering the house and kill mosquitoes before feeding. The fraction of mosquitoes that survive can be blocked by the ITN, which may also kill a subset of the blocked fraction. Those mosquitoes who survive the feeding attempt may be killed by IRS post-feed. This is how deterrent and toxic effects of multiple interventions can be represented simultaneously.
To interact with these parameters and visualize the workings of this microsolver, see the decision tree visualization below:
To get started, press the play button. You can also pause the visualization at any time. Parameters in blue are vector species parameters, while parameters in green are types of campaign interventions. Information on these parameters can be found in Configuration parameters and Campaign parameters. The two pink points on the tree illustrate when transmission of malaria parasites is possible.
When the simulation starts, the initial mosquito population is set at 100 individuals. The starting population for day two has an initial seeding of 50 mosquitoes, and also includes all mosquitoes that either live without feeding or feed and oviposit. The simulation includes parameters that determine the lifespan of mosquitoes and the time it takes for oviposited eggs to hatch and mature to adulthood. As time progresses, the population will be comprised of only mosquitoes that are generated through the oviposition cycle in the model.
The counters on the right side of the visualization keep track of current and total mosquitoes that have “spawned” (generated in the simulation), died, lived without feeding, and fed and oviposited.
As an example, let’s simulate Anopheles gambiae. Set Life_Expectancy to 10 (most are thought to live approximately 1-2 weeks in nature), Egg_to_Adult to 5 (this is their minimum duration in the aquatic phase), Days_between_Feeds to 3, and Anthropophily and Indoor_Feeding_Fraction to 0.8. These mosquitoes prefer to primarily feed on humans, and preferentially feed indoors. Now, by changing the interventions, you can see how effective interventions (or combinations of interventions) need to be in order to disrupt (and reduce) mosquito feeding and oviposition. Note that the slider bars for interventions range from 0 - 1, with 1 conferring 100% effectiveness. When mosquito ecology is sufficiently disrupted, malaria transmission can be controlled. You can also manipulate the species parameters to see how mosquito ecology impacts the need for particular types of interventions.
If you are interested in simulating other mosquito species, more information on their relevant attributes can be found in the articles Made-to-measure malaria vector control strategies: rational design based on insecticide properties and coverage of blood resources for mosquitoes, by Killeen et al., 2014, Malaria Journal 13:146, and A global bionomic database for the dominant vectors of human malaria, by Massey et al., 2016, Nature.
Transmission between humans and vectors¶
Transmission between humans and vectors can only occur when mosquitoes successfully feed on humans (see Modeling feeding cycle outcomes). Infectious humans can infect susceptible mosquitoes when mosquitoes ingest gametocytes during the blood meal. Conversely, infectious mosquitoes can infect susceptible humans by injecting sporozoites into the blood during feeding. Infectiousness of individuals to mosquitoes is proportional to the number of mature gametocytes absorbed in a blood meal, but modulated by a factor that inactivates gametocytes at high cytokine densities. Peak infectiousness typically follows peak parasitemia by about ten days. See the Malaria infection and immune model for more information regarding within-host parasite dynamics, and the figure on the parasite life-cycle in the Malaria disease overview for more information on the parasite life stages.
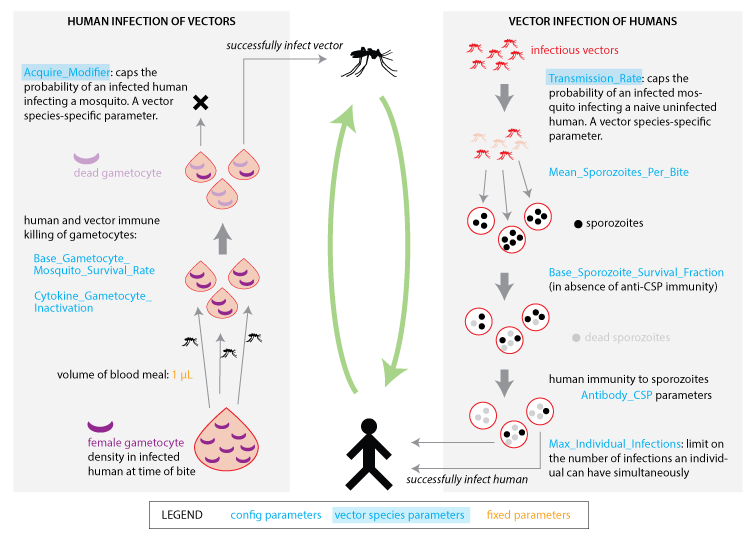
Transmission between humans and vectors¶
Relevant IDM publications¶
Eckhoff, 2013. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg. 88(5):817-27
Eckhoff, 2011. A malaria transmission-directed model of mosquito life cycle and ecology. Malaria Journal. 10:303