Malaria model¶
The malaria model inherits the functionality of the vector model and introduces human immunity, within-host parasite dynamics, effects of antimalarial drugs, and other aspects of malaria biology to simulate malaria transmission. For example, individuals can have multiple infections and both innate and adaptive responses to antigens. To use the malaria model, set the configuration parameter Simulation_Type to MALARIA_SIM.
Discrete and continuous processes¶
The model implements a hybrid of discrete and continuous processes that work together to capture system latencies and discrete events inherent in the population dynamics of humans and mosquitoes, and parasites. Discrete events, such as latencies in the infected hepatocyte stage, the length of the asexual cycle from merozoite invasion to schizont rupture, and gametocyte maturation have particular time durations. State changes occur after the completion of the required amount of time (with specific time durations set for each state). See the figure of the Plasmodium life-cycle in Malaria disease overview for more information regarding the life-cycle stages used in this example.
Other processes, such as the decay of antibodies and the clearance of parasites, are represented by continuous-time processes. These are solved with a one-hour time step Euler method. All parasite quantities, such as the number of hepatocytes, number of merozoites, number of infected red blood cells of each antigenic variant, and gametocytes of each stage are represented as discrete integers. The infection is not cleared until each category is reduced to zero. This allows resolution of model dynamics at subpatent levels.
If using the modified mosquito cohort model introduced with the vector model, it allows temperature-dependent progression through sporogony, even with a different mean temperature each day, with no mosquitoes passing from susceptible to infectious before the full discrete latency.
Model components¶
The malaria model is complex, with numerous configurable parameters. The following network diagram breaks down the model into various model components, and illustrates how they interact with one another. The components on the network diagram correspond to the structural components listed below. Note that there is not perfect overlap between the labels on the network diagram and the structural components; this is because the network is drawn with increased detail in order to provide clarity in how the model functions and the components interact. The following pages will describe in detail how the structural components function.
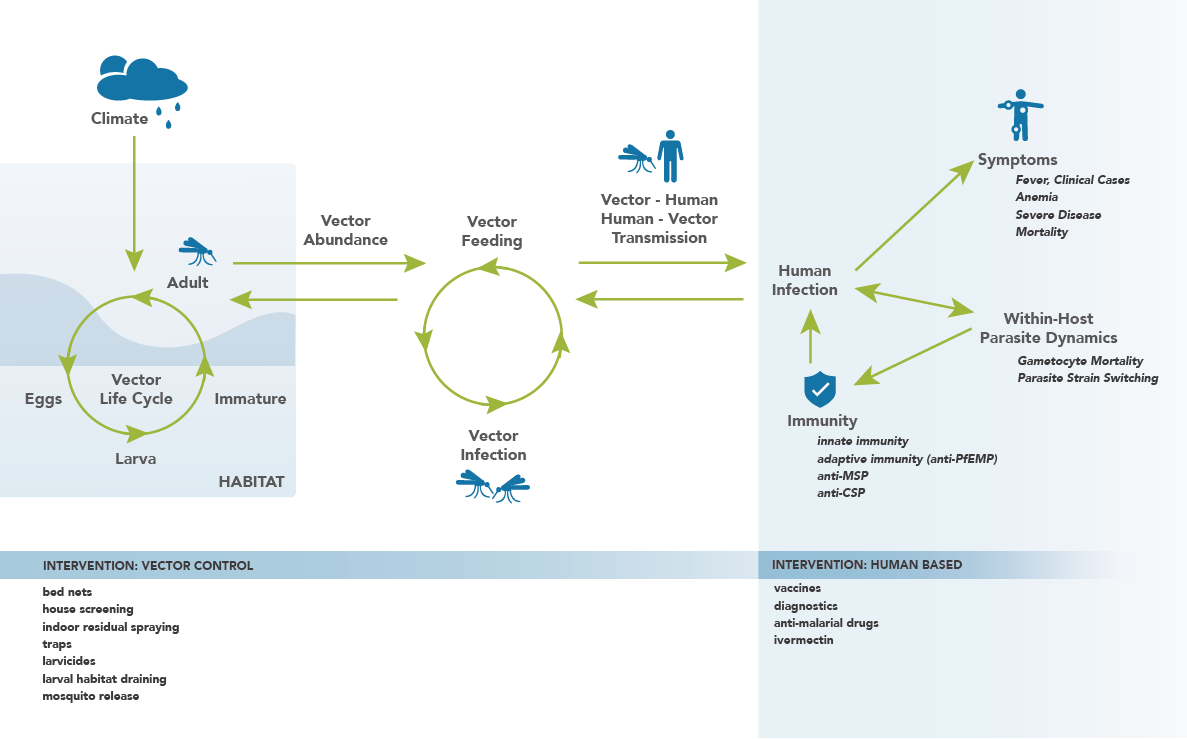
Network diagram illustrating the malaria model and its constituent components.¶
As new technology arises and novel types of interventions become available, it will be relatively seamless to integrate them into the current model structure. For example, as genetic technology improves and testing the utility of utilizing GM mosquitoes (such as a gene drive mosquito) in control efforts becomes important, EMOD will have no difficulty integrating this novel intervention into simulations.
The following pages describe the structure of the model and explore each of the model components. Additionally, the specifics of the model are discussed in detail in the articles Eckhoff, Malaria Journal 2011, 10:303; Eckhoff, Malaria Journal 2012, 11:419; Eckhoff, PLoS ONE 2012, 7(9); and Eckhoff, Am. J. Trop. Med. Hyg. 2013, 88(5).