Antimalarial drugs#
Antimalarial drugs are a powerful tool for malaria control and elimination. Modeling mass antimalarial campaigns can elucidate how to most effectively deploy drug-based interventions and quantitatively compare the effects of cure, prophylaxis, and transmission-blocking in suppressing parasite prevalence.
Individuals in a simulation can receive antimalarial drugs as treatment for clinical or severe malaria or during a mass drug administration (MDA) campaign. The following information explains how to configure antimalarial drug parameters in a configuration file. For a complete list of configuration parameters that are used in the malaria model, see the Configuration parameters.
To specify where, when, and to whom the antimalarial drugs are administered, you must use an AntiMalarialDrug, AdherentDrug, or MultiComboPackDrug intervention in the campaign file. See Campaign parameters for more information.
Modeling a box profile of antimalaria drug pharmacokinetics#
Pharmacokinetics (PK) of antimalarial drugs can be modeled with a box profile or with a double exponential decay. Pharmacodynamics (PD) of antimalarial drugs are modeled as an on/off switch for box PK or with a simple sigmoid for double exponential PK. If multiple drugs are given in a treatment, each drug is considered independently for both PK and PD.
To model a box PK, set the parameter PKPD_Model to FIXED_DURATION_CONSTANT_EFFECT. Drugs will have full killing efficacy for the duration set by Drug_Decay_T1. Dosing multiple times leads to multiple pulses of drug killing if dose separation is longer than Drug_Decay_T1. If dose separation (Drug_Dose_Interval) is shorter than Drug_Decay_T1, the box time is reset by each new dose such that drug killing is switched off after time Drug_Decay_T1 after the last dose.
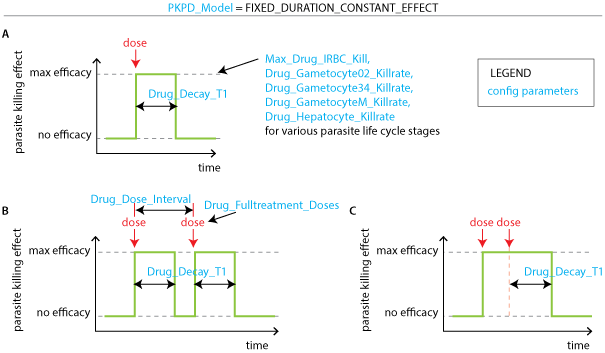
Modeling drug killing effects with a box profile. (A) A single dose of drug kills parasites at maximum efficacy for duration set by Drug_Decay_T1. (B) A drug given in multiple doses results in multiple parasite killing pulses of duration Drug_Decay_T1 if the dose separation Drug_Dose_Interval is longer than Drug_Decay_T1. (C) If a drug’s dosing regimen results in doses taken before time Drug_Decay_T1 has elapsed, the drug kills parasites at maximum efficacy the entire time between the first dose and time Drug_Decay_T1 after the last dose.#
The following example provides the syntax:
{
"Malaria_Drug_Params": [
{
"Name": "Artemether_Lumefantrine",
"PKPD_Model": "FIXED_DURATION_CONSTANT_EFFECT",
"Drug_Cmax": 1000,
"Drug_Decay_T1": 1,
"Drug_Decay_T2": 1,
"Drug_Dose_Interval": 1,
"Drug_Fulltreatment_Doses": 3,
"Drug_Gametocyte02_Killrate": 2.3,
"Drug_Gametocyte34_Killrate": 2.3,
"Drug_GametocyteM_Killrate": 0,
"Drug_Hepatocyte_Killrate": 0,
"Drug_PKPD_C50": 100,
"Drug_Vd": 10,
"Max_Drug_IRBC_Kill": 4.61,
"Bodyweight_Exponent": 0
}
]
}
Modeling a double exponential PK#
To model a double exponential PK, set the parameter PKPD_Model to CONCENTRATION_VERSUS_TIME. The double exponential PK approximates a one- or two-compartment PK model. To model a drug with 1-compartment PK, set Drug_Decay_T2 equal to Drug_Decay_T1 (Drug_Vd becomes irrelevant). For PD, the Drug_PKPD_C50 determines the drug concentrations where parasite killing is effective (drug concentration > C50) and ineffective (drug concentration < C50). EMOD sets the shape of the parasite killing curve based on parasite kill rate and C50. Drug_Cmax, Drug_Decay_T1, Drug_Decay_T2, Drug_Vd, and Drug_PKPD_C50 together determine when the drug is effectively killing parasites. Changing each of these parameters will affect how long the parasites are exposed to a strong killing effect. Adding additional doses will also increase the duration of the parasite- killing window.
Children can be dosed with a fraction of the adult dose according to Fractional_Dose_By_Upper_Age, where each dose fraction is paired with the maximum age of children receiving that dose. Depending on specific PK characteristics of each drug, bodyweight may affect the rate of drug clearance. In the double exponential PK model, bodyweight is directly determined by age, and Drug_Cmax is multiplied by the inverse of the bodyweight raised to the power of Bodyweight_Exponent. See the paper on drug PK and PD ( Mass campaigns with antimalarial drugs: a modelling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination) for more details on how to set PK and PD parameter values in EMOD.
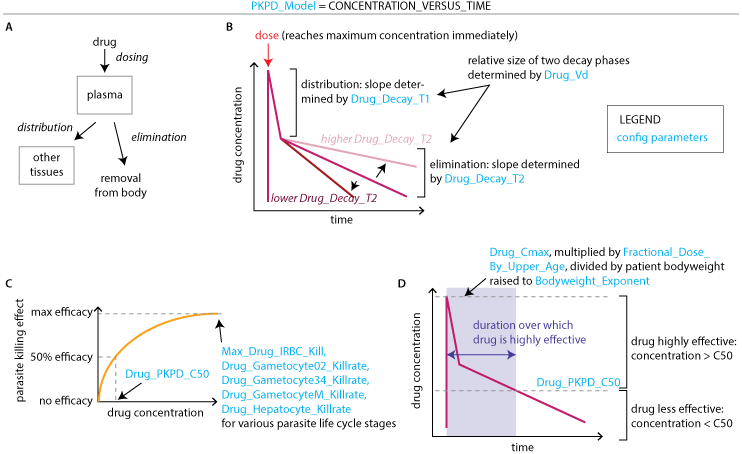
Modeling drug killing effects with a double exponential PK and sigmoid PD. (A) Two-compartment PK models include both distribution and elimination of drug. One-compartment models do not have a distribution phase. (B) A double exponential PK approximates the distribution and elimination phases of a two- compartment model. (C) Parasite killing is determined by drug concentration, Drug_PKPD_C50, and parasite stage- specific maximum kill rates. (D) PK and PD together determine the duration over which a dose of drug is effective.#
Relevant IDM publications#
Antimalarial drugs#
Bellinger, et al., 2016. Oral, ultra–long-lasting drug delivery: Application toward malaria elimination goals. Science Translational Medicine. 8(365).
Gerardin, Eckhoff and Wenger, 2015. Mass campaigns with antimalarial drugs: a modeling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination. BMC Infectious Diseases. 15:144
Vaccines#
Penny, et al., 2016. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. The Lancet. 387(100016): 367-375
McCarthy, Wenger, Huynh and Eckhoff, 2015. Calibration of an intrahost malaria model and parameter ensemble evaluation of a pre-erythrocytic vaccine. Malaria Journal. 14:6
Wenger & Eckhoff, 2013. A mathematical model of the impact of present and future malaria vaccines. Malaria Journal. 12:126
